1.SCOPE
This Article standard covers the requirements of aluminium and aluminium alloy-bare / coated / laminated foil for Pharmaceutical packaging applications. It is applicable for 0.020mm (20μm) to 0.040mm (40μm) foil thickness.
2.REFERENCES
The following standards contain provisions, which through reference in this text constitute provision of this standard.
At the time of publication, the editions indicted were valid. All standards are subject to revision and parties to agreements based on this standard are encouraged to investigate the possibility of applying the most recent editions of the standards indicated below.
11 AVERAGE THICKNESS
11.1 The determination of average thickness shall be carried out using a method giving repeatable results.
11.2 In case of dispute, the average thickness may be determined by the gravimetric method, based on weighing a sample of 100mm x 100 mm area, shall be dried and weighed on a balance, accurate to at least 0.5 mg
Thickness of the foil, in mm = w/27.1
12.1 Pharma Laminate - It will have one side laminated with 35 gsm LDPE. Non laminated side shall have primer coating with Shellac or shall be printed as desired by the purchaser.
12.2 Coated Blister pack foil – It shall have one side of the aluminium foil coated with heat sealant lacquer with a coating weight in the range of either 4-6gsm or 6-8gsm depending on mutual agreement between purchaser and supplier. Primer coating with Shellac or printing shall be done on the non-heat seal side.
12.3 Winding - Coated Blister pack foil shall be so wound so that the primer-coated side is on the outside surface.
13 DIMENSIONS AND TOLERANCES
13.1 Unless otherwise agreed, the thickness tolerance for bare foil shall be +/- 8% and the thickness tolerance for laminated products (including the foil and lamination together) shall be +/- 8 percent.
13.2 Unless otherwise stated, the width tolerances shall be as given in Table 3.
This Article standard covers the requirements of aluminium and aluminium alloy-bare / coated / laminated foil for Pharmaceutical packaging applications. It is applicable for 0.020mm (20μm) to 0.040mm (40μm) foil thickness.
2.REFERENCES
The following standards contain provisions, which through reference in this text constitute provision of this standard.
At the time of publication, the editions indicted were valid. All standards are subject to revision and parties to agreements based on this standard are encouraged to investigate the possibility of applying the most recent editions of the standards indicated below.
IS No.
|
Title
|
737: 2008
|
Wrought
aluminium and aluminium alloy sheet and strip for general
engineering
purposes ( fourth revision )
|
5047 (Part 1):1986
|
Glossary of
terms relating to aluminium and aluminium alloys: Part 1
Unwrought
and wrought metals (second revision )
|
10259: 1982:
|
General
conditions for delivery and inspection of aluminium and
aluminium
alloy products
|
13237: 1991:
|
Metallic
foil- -Tension testing
|
3. TERMINOLOGY
For the purpose of this standard, the following definitions in addition to those given in IS 5047 (part 1) shall apply.
3.1 Bare Blister pack foil – Bare foil (of thickness 20-25μ without any lamination or coating) used for blister pack application for pharmaceutical packaging usually after coating.
3.2 Coated Blister pack foil – Bare foil (of thickness 0.20-25μ) with one side coated with Heat seal lacquer and the other side with primer or printed used for blister pack application in pharmaceutical packaging.
3.3 Bare Pharma Strip pack foil – Bare foil (of thickness 30-40μ without any lamination or coating) used for strip pack application for pharmaceutical packaging.
3.4 Pharma laminate – Laminated foil (with foil thickness of 30-40μ) with one side laminated and other side coated with primer or printed used for strip pack application for pharmaceutical packaging
4 MANUFACTURE
Unless otherwise specified, the production and manufacturing processes shall be left to the discretion of the manufacturer.
PINHOLE COUNT
Pinhole count will be mutually agreed upon between purchaser and supplier. However, a guideline for pinhole count is given in Table 1.
6 FREEDOM FROM DEFECTS
The foil shall be well finished, uniform in quality, free from splits, slivers, wrinkles, ragged edges and oil staining. If supplied in coated / laminated condition the coating / lamination shall be uniform. There shall be no delaminated areas.
7 MATERIAL
7.1 The material used for aluminium and aluminium alloy foil shall conform to the chemical composition of the Grades 19000, 19500, 19600,31000 and 40800 of IS 737.
7.2 Unless otherwise specified by the purchaser, Bare Blister pack foil shall be supplied in “as rolled” condition without a final annealing
treatment.
Bare Pharma Strip pack foil shall be supplied in fully annealed condition. Coated Blister pack foil shall be supplied with one side of as rolled foil (without a final
anneal) coated with heat seal lacquer and the other side coated with shellac or printed.
Pharma laminate shall be supplied with one side of fully annealed foil laminated with 35gsm LDPE and other side coated with Shellac or printed
8 SUPPLY OF MATERIAL
General requirements relating to the supply of aluminium and aluminium alloy foil shall conform to IS 10259.
9 LUBRICANTS
As the foils are to be used in pharmaceutical applications they shall be produced with rolling oils/lubricants which do not contain substances which are injurious to health or have any deleterious effect on the flavour, odour or appearance of pharmaceutical products.
10 PREFERRED THICKNESSES
Unless otherwise stated, the preferred thickness shall be as given in Table 2.
11 AVERAGE THICKNESS
11.1 The determination of average thickness shall be carried out using a method giving repeatable results.
11.2 In case of dispute, the average thickness may be determined by the gravimetric method, based on weighing a sample of 100mm x 100 mm area, shall be dried and weighed on a balance, accurate to at least 0.5 mg
Thickness of the foil, in mm = w/27.1
- where w is the mass of the foil sample ( 100 mm x 100 mm) in g.
12.1 Pharma Laminate - It will have one side laminated with 35 gsm LDPE. Non laminated side shall have primer coating with Shellac or shall be printed as desired by the purchaser.
12.2 Coated Blister pack foil – It shall have one side of the aluminium foil coated with heat sealant lacquer with a coating weight in the range of either 4-6gsm or 6-8gsm depending on mutual agreement between purchaser and supplier. Primer coating with Shellac or printing shall be done on the non-heat seal side.
12.3 Winding - Coated Blister pack foil shall be so wound so that the primer-coated side is on the outside surface.
13 DIMENSIONS AND TOLERANCES
13.1 Unless otherwise agreed, the thickness tolerance for bare foil shall be +/- 8% and the thickness tolerance for laminated products (including the foil and lamination together) shall be +/- 8 percent.
13.2 Unless otherwise stated, the width tolerances shall be as given in Table 3.
14 MECHANICAL PROPERTIES
14.1 BURSTING STRENGTH
Bursting strength for bare strip pack foil and pharma laminate shall be as per table 4. Note -Bursting strength is only applicable for bare strip pack foil or pharma laminate. It is not applicable for bare blister pack foil or coated blister pack foil.
Table 4 Bursting Strength
14.2 PEELING STRENGTH (For Pharma Laminate)
Peel strength is only applicable for Pharma Laminate and shall be as per Table 5.
14.3 Sealing Strength (For Coated Blister Pack foil and Pharma Laminate)
Sealing strength is only applicable for Coated Blister Pack Foil and Pharma Laminate and shall be as per Table 6.
15 SURFACE CONDITION
15.1 For Coated Blister Pack Foil one of the foil surfaces is bright and the other surface is matte.
15.2 For Pharma Laminate either one of the foil surfaces is bright and the other surface matte or both the surfaces may be bright depending on requirements of the purchaser.
16 SAMPLING
16.1 Unless otherwise agreed to between the purchaser and the manufacturer the following procedure and the criteria for conformity shall apply.
16.2 In a consignment the foils of same width and thickness and of the same surface condition and manufactured by a single firm under essentially similar conditions of production shall be grouped together to constitute a lot.
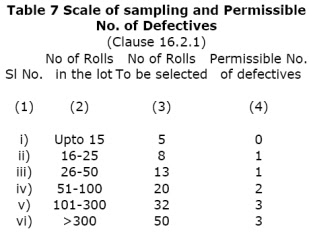
- 16.2.1 Tests for determining the conformity of the lot to the requirement of this standard shall be carried out on each lot separately. The number of rolls of foils to be selected for this purpose at random over the whole lot shall be in accordance with col 2 and 3 of Table 7
- 16.2.2 All the rolls shall be individually examined for manufacturing defects, surface defects and dimensional tolerances. A sample failing to meet any one of these requirements shall be called defective. The lot shall be considered as conforming to the corresponding requirements of this standard. If number of defectives satisfy the freedom from defects and dimensions in less than or equal to the permissible number given in col 4 of Table 7.
17 ORDERING INFORMATION
The order shall include the following information:
a) Quantity in kg;
b) Nominal thickness;
c) Foil size;
d) Dimensions of rolls (outside diameter, in mm);
e) Type and inside diameter of the core in mm; length of core (if different from the width of the rolls);
f) Surface condition; and
g) Packing mode
18 MARKING
18.1 Each package of bare or converted aluminium foil may be suitably marked for identification with the name of manufacturer, grade, condition of the material, batch No. and date of manufacture.
- 18.1.1 The foil package may also be marked with the Standard Mark.
- 18.1.1.1 The use of the Standard Mark is governed by the provision of the Bureau of Indian Standards Act, 1986 and the Rules and Regulations made thereunder.
- The details of conditions under which the licence for the use of the Standard Mark may be granted to manufacturers or producers may be obtained from the Bureau of Indian Standards.